Monthly Gene Therapy Business Review - January 2018
This newsletter provides commercial insights into the ongoing development of the gene therapy market. The most noteworthy stories appear at the top along with our commentary.
1) Still Learning What We Don’t Know: Gene Therapy Market Impacted by Safety Concerns
Extreme market response to postulated gene therapy immunity and the unsettling results of research just made public by an industry pioneer remind us that the gene therapy market is still in its infancy. We still don’t know what we don’t know, particularly when it comes to long-term outcomes and adverse events. Inherent risk and unknowns affect both how and how quickly early therapies will be adopted and with which patients. Effective patient segmentation and communication are key and shouldn’t be underestimated even with successful clinical results.
Two unrelated reports emerged this month questioning the safety of gene therapy treatments, one of which sent Intellia, Editas, and CRISPR stocks tumbling $500 million (temporarily). This extreme reaction to the news that many people could be immune to CRISPR-Cas9 technology demonstrates the immaturity of the gene therapy market and how much we still don’t know.
Another report, authored by Dr. James Wilson, revealed that nerve and liver damage occurred in animals who received gene therapy treatment for SMA. Humans have responded well to gene therapy for SMA but Dr. Wilson’s caution to carefully monitor subjects underscores the fact that we still don’t know the long-term effects of gene therapy treatment or the potential result of overdosing.
One possible solution to safety concerns comes from an unexpected source: Microsoft. The company has developed a new tool, Elevation, that uses artificial intelligence to predict off-target effects. This tool may identify patients who would respond optimally to certain gene therapies and track patients and treatment reactions over time.
BioRxiv: Identification of Pre-Existing Adaptive Immunity to Cas9 Proteins in Humans
Human Gene Therapy: Severe toxicity in nonhuman primates and piglets following high‐dose intravenous administration of an AAV vector expressing human
Nature: Prediction of off-target activities for the end-to-end design of CRISPR guide RNAs
Genetic Engineering and Biotech News: FDA Imposes Clinical Hold on Bellicum Lead Candidate BPX-501
2) Headline “Gene Therapy Could Make Cancer Care More Unequal” Probably Not True
A recent article in MIT Technology Review used that headline to grab attention, but we maintain that gene therapy is not more unequal than other types of treatments for rare diseases, especially when other therapies cost $475,000 and many patients already travel to leading cancer centers for treatment. Access to care in the US is a system problem, not just a gene therapy problem.
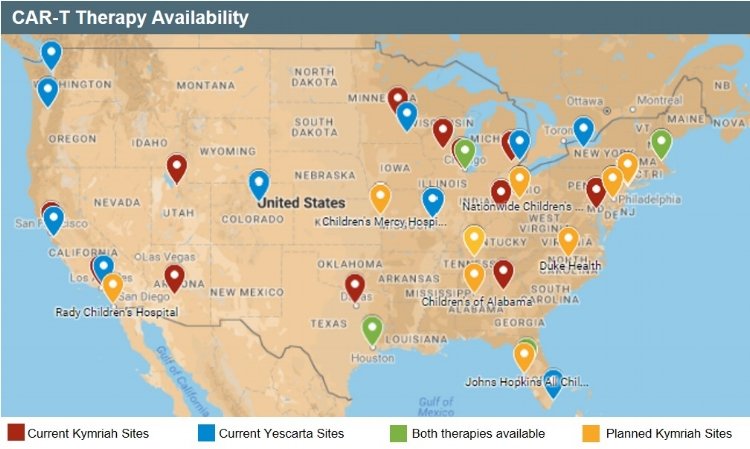
Due to the cost and required expertise for effective treatment, most companies commercializing gene therapies will rely on a “centers of excellence” distribution model. An interactive map in the article demonstrates the treatment sites for Kymriah and Yescarta.
In rolling out Kymriah, Novartis underscored its achievement developing a “reproducible product” after manufacturing CAR-T cells for more than 250 patients from 11 countries. This is proof that centralized manufacturing can work for gene therapy treatments.
MIT Tech Review: Gene Therapy Could Make Cancer Care More Unequal, and This Map Shows Why
Genetic Engineering and Biotech News: Cell Therapy Manufacturing: All Signs Point to Commercialization
3) China’s Quest for Gene Therapy Success
Three news articles emerged this month that paint a picture of aggressive Chinese development of gene therapy treatments and technologies. A shorter regulatory process along with willingness to skirt potential ethical issues have made China a gene therapy hotspot. While it is unlikely that gene therapy developers in the United States will face future commercial challenges from China, the speed with which China is advancing gene therapy will be important to monitor.
The Wall Street Journal: China, Unhampered by Rules, Races Ahead in Gene-Editing Trials
The Wall Street Journal: China Breaks a Cloning Barrier: Primates
Washington Post: Targeting Biotech’s 1%, China Speeds Into Gene Therapy
4) Answering ICER: Spark CEO Offers Rationale for Luxturna Price
This case serves as a good reminder that gene therapy stakeholders—especially developers, investors, and payers—should adopt a broad perspective as the way we value therapies continues to evolve.
Institute for Clinical and Economic Review (ICER) did not surprise anyone with their updated report on Spark Therapeutics’ $850k price tag for Luxturna, revealed before #JPMorgan18. In its report, ICER concludes that Luxturna should be priced 75-82% less to be cost effective for a 15-year-old patient, the average patient age in Spark’s clinical trials. The report also noted that when accounting for all savings from indirect “societal benefits,” Luxturna should be priced 50-57% less.
Spark CEO Jeff Marrazzo’s interview with Xconomy provides insight into what factors the company used to determine Luxturna’s value and to price accordingly.
Thinking short term: “We didn’t necessarily grab all of the value, and we went above what payers were positioning as affordability. But we came in to a point where, most importantly, I believe we can drive access for patients.”
Using non-traditional economic measures: “There were about 25 court cases in states that release award information for people who either partially or fully lost their sight. The awards were $250,000 on the low end and $4 million on the high end of those cases, with an average at about the $1 million mark ... I think that’s helpful in showing how our society values sight.”
Thinking long term: “I feel a certain responsibility to make sure we have commercial success, but I also felt we should do it in a way I think the world should look at in the future … when you start talking about hemophilia, Pompe disease, AveXis with their [spinal muscular atrophy] product, which may be replacing a chronic drug in nusinersen (Spinraza), there are cost offsets. In one of our hemophilia trials, we had 10 patients that take, on average, $406,000 per year of [blood-clotting factor replacement drugs].”
News and Updates
Global
Globe Newswire: Spark Therapeutics Enters into a Licensing and Supply Agreement for Investigational Voretigene Neparvovec Outside the U.S. with Novartis
CAR-T Updates
US News: Could Gene Therapy Someday Eliminate HIV? (see also PLOS Pathogens: Long-term persistence and function of hematopoietic stem cell-derived chimeric antigen receptor T cells in a nonhuman primate model of HIV/AIDS)
Genetic Engineering and Biotech News: Kite Earns Patent for Method to Increase Efficacy of CAR-T
CRISPR Updates
MIT Tech Review: U.S. Doctors Plan to Treat Cancer Patients Using CRISPR
G enetic Engineering and Biotech News: Nonviral Gene Therapy Platform Delivers CRISPR/Cas9 to Tumors
Funding, Launch, and Partnership News
BioSpace: REGENXBIO Banks on Strong Gene Therapy Pipeline and Potential Partnerships
Genetic Engineering and Biotech News: Sangamo, Pfizer Partner on Gene Therapy for ALS, FTLD
Clinical Stage Research Updates
Singularity Hub: IONIS antisense drug advancing in Huntington’s Disease after Phase 1/2a study success
Pre-Clinical Stage Research Updates
Nature: Targeted repair of heart injury by stem cells fused with platelet nanovesicles
Fierce BioTech: Atlas-backed Generation Bio promises ‘druglike’ gene therapy
Medical News Today: Diabetes: Can gene therapy normalize blood glucose levels?
Interesting News
MIT Tech Review: Meet the Woman Using CRISPR to Breed All-Male “Terminator Cattle”
Oregon Live: Climate change might kill off chocolate by 2050, so scientists turn to CRISPR gene-editing technology
Sign Up for More
If you would like to subscribe to RxC International’s Monthly Gene Therapy Business Review, sign up here.
About RxC International
RxC International is a premier life sciences management consulting firm. RxC collaborates with clients to identify and develop growth opportunities. The firm leverages consulting partners and advisers to combine strategic and operational expertise to bring multiple perspectives to every engagement. The firm has deep expertise in corporate strategy, new product strategy, and commercial excellence.