RxC ProPlan™
New Product Plan to Maximize Potential
The early-stage dilemma in new product planning
As soon as the discovery team identifies a target for a particular disease, it's critical to start understanding the potential of this target before embarking on the journey of further development. The cross-functional teams have to evaluate the opportunity to rationalize investments. The scientific opportunity must align with the commercial potential to advance a new product for development.
Key Drivers of New Product Assessment
As part of the new product evaluation, biopharma companies must address the following questions to gain a deeper understanding of the opportunity.
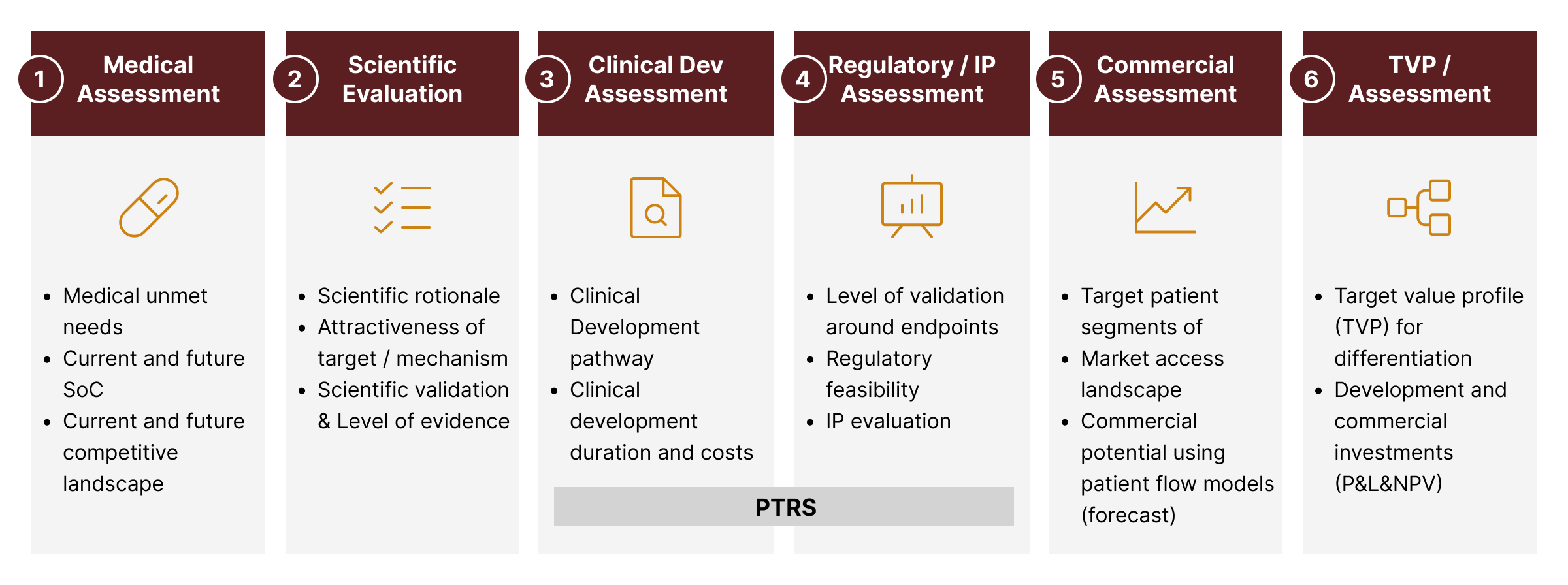
a) Scientific and Medical Rationale
What level of target validation is available?
What is the unmet medical need addressed?
What is the current and future Standard of Care?
b) Clinical Development Pathway
What is the clinical development pathway?
What are the clinical study requirements?
What is the clinical trial feasibility and probability of success?
c) Regulatory Feasibility
What is the regulatory pathway?
What are the primary and secondary endpoints, including PROs?
What is the IP strategy?
What is the regulatory feasibility and probability of success?
d) Technical Feasibility
What is the tech feasibility of developing the drug?
What are the tech ops / CMC requirements?
What are the supply chain requirements?
e) Commercial Attractiveness
What is the commercially attractive profile (TVP)?
What is the commercial attractiveness (market access and peak revenue)?
What investments are required to maximize RoI?
Our ProPLAN™ Solution will Help with the New Product Investment Decisions
ProPLAN leverages best practices from more than 100 new product assessments to guide biopharma companies in developing new product strategies and guiding them through the decision-making process around whether to invest. As highlighted below, a new product strategy must address medical, scientific, clinical, tech ops, regulatory, and commercial opportunities. We work closely with the medical, clinical, regulatory, tech ops, and early commercial teams on these new product assessments.
Our ProPLAN™ Proprietary Framework for New Product Assessments:
RxC Differentiation…
We have Hands-on Experience in Guiding New Product Investment Decisions.
Over the last decade, we have completed over 100 new product assessments for pharma and biotech companies. In addition, we have helped several biopharma companies with go/no-go decisions as executives within the industry directly responsible for investment decisions and as consultants advising on how best to navigate through the complexity of these decisions. We are uniquely positioned to collaborate with biopharma teams to bring the right insight to make informed decisions.
Latest Publications on Product Commercialization
Clients and Case Studies
Check out examples of product commercialization work that our team has successfully completed for leading pharma and biotech companies.
RxC International has worked with a number of leading biopharma companies on commercialization initiatives. Our clients range from Fortune 100 companies to small cap companies in the life sciences sector.
RxC has worked with clients from around the world to successfully develop and commercialize dozens of products. Below is a representative list of projects to which we have applied our frameworks.