Monthly Gene Therapy Business Review - February 2018
This newsletter provides commercial insights into the ongoing development of the gene therapy market. The most noteworthy stories appear at the top along with our commentary.
Top Stories:
Lessons Learned from Hepatitis C: Intense Competition Requires Careful Commercial Planning for Sickle Cell Gene Therapies
Gene Therapy IP, Manufacturing, and Delivery Pathways Still Evolving
FDA Moving Forward with its Role in Advancing a Modern Framework for Gene Therapy
1) Lessons Learned from Hepatitis C: Intense Competition Requires Careful Commercial Planning for Sickle Cell Gene Therapies
Multiple gene therapy companies are developing treatments for 4+ million worldwide sickle cell patients (~100k in the US and ~130k in the EU). With comparable development timetables and Probabilities of Technical and Regulatory Success (PTRS) due to similar technology and development pathways, companies should anticipate multiple market entries. Furthermore, clear adoption challenges for even the first to market will require careful planning and comprehensive market analyses to succeed.
The Hepatitis C market offers a potential peek into the future of sickle cell treatments. Long known to be one of the largest blockbuster opportunities, HepC attracted enormous R&D investment and saw a bevy of new products launched including multiple products that cure the disease. Despite the medical triumph, commercialization and the competitive market have presented enormous challenges to innovator companies for maximizing economic value.
The most recent challenge is the newly-announced $26.4k pricing per treatment course for AbbVie's Mavyret (significantly lower than Gilead's launch price of $84-95k per treatment course for its products Solvadi and Harvoni). That along with other market challenges have brought Gilead's worldwide revenue down from $19b in 2015 to approximately $5b in 2017, despite millions of potential untreated HepC patients.
Stay tuned for RxC’s analysis of applying commercial lessons learned from Hepatitis C to sickle cell gene therapies.
Reference Articles
STAT: More than a half-dozen teams are pursuing genetic therapies for sickle cell
FiercePharma: Dogged by AbbVie's new share-stealing Mavyret, Gilead's hep C forecast comes in far short
Huffington Post: How Black Communities Could Better Help Sickle Cell Patients
2) Gene Therapy IP, Manufacturing, and Delivery Pathways Still Evolving
Gene therapy is still a nascent segment of healthcare and continues to experience growing pains, particularly in regards to intellectual property, manufacturing, and delivery. Here are the latest developments RxC International is monitoring:
IP: Kite/Gilead gained potential commercial advantage by patenting a specific preconditioning protocol that could cause IP trouble for its competitors.
Manufacturing: Sorrento’s non-viral, "off-the-shelf" CAR-T technology could translate into faster development timelines, more cost-effective cGMP manufacturing and possible removal of the regulatory requirement to follow patients for 15 years post treatment.
Delivery: Sangamo’s multi-decade effort in developing zinc fingers (an alternative to CRISPR gene therapy delivery technology) is beginning to pay off. “We can now target any nucleotide in the genome,” says President/CEO Sandy Macrae.
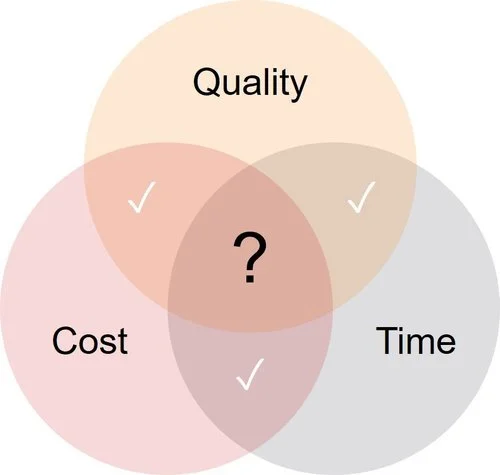
Time – Quality – Cost: You only can control 2 of the 3 currently.
— Bill Lundberg, Alliance for Regenerative Medicine Conference, #JPMorgan18
Reference Articles
Kite Earns Patent for Method to Increase Efficacy of CAR-T (Genetic Engineering and Biotech News)
Sorrento Therapeutics to Present Potential “Game-Changer” Non-Viral CAR-T Technology for Autologous and Allogeneic (off-the-shelf) CAR-T Therapies (Globe Newswire)
Lost in the CRISPR Hype, a Gene-Editing Giant (Sangamo) Is Fighting Back (Healthcare Analytics News)
(Sangamo) 2nd Man Has Gene Editing; Therapy Has No Safety Flags So Far (US News)
3) FDA Moving Forward with its Role in Advancing a Modern Framework for Gene Therapy
FDA approval of a direct-to-consumer test for rare BRCA gene mutations may open the door to improved early diagnosis/monitoring of genetically-based diseases. Dr. J. Leonard Lichtenfeld, deputy chief medical officer for the national office of the American Cancer Society, says he is generally encouraged by the "slow and steady and deliberate movement toward more open access to genetic information" and believes that "eventually, we will get to the point where testing for other cancers [risks] will be routine."
23andMe received FDA approval for its direct-to-consumer BRCA gene mutation test. Women with one of these mutations have a 45-85% chance of developing breast cancer by age 70. While some view this test as beneficial to consumers, others caution that its narrow scope (detecting only 3 of more than 1,000 BRCA mutations) may create a false sense of security in women who test negative.
Reference Articles
STAT: FDA approves first direct-to-consumer test for breast cancer risk
CNN: FDA approval of self testing for breast cancer genes comes with cautions
Forbes: 23andMe Stops Offering Genetic Tests Related To Health (2013)
News and Updates
CRISPR Updates
American Council on Science and Health: New Journal Is All CRISPR, All The Time
Funding, Launch, and Partnership News
PharmaTimes: AbbVie, Voyager to develop single gene therapy for Alzheimer’s
FierceBiotech: Sanofi drops Voyager’s phase 2/3 Parkinson’s gene therapy (October, 2017)
FierceBiotech: Sanofi embarks on an $845M gene therapy R&D odyssey with Voyager (February, 2015)
Clinical Stage Research Updates
University of Florida Health: Gene therapy for pulmonary dysfunction in Pompe disease is found safe during first human trial
Pre-Clinical Stage Research Updates
Genetic Engineering and Biotech News: Reactivating a Dormant X: A New Strategy to Treat Rett Syndrome
Other News
Gizmodo: The Search for the Olympian Gene
About RxC International
Understanding the subtleties of gene therapies is critical for bringing these treatments to market. RxC International has extensive experience successfully commercializing and launching new drug products, developing innovative solutions, realizing a product's best potential, and working across organizations to achieve common goals.
If you would like to subscribe to receive RxC International’s Monthly Gene Therapy Business Review, sign up here.