RxC ProComS™
New Product Commercialization Strategy to Maximize Potential
Looking for the Right Plan to Commercialize a New Drug?
As a biopharma company, all essential commercial preparations must be commenced years in advance of your product gaining regulatory approval. Unique strategies are needed to engage patients, providers, and payers—especially given their diverse needs within our healthcare system. Early efforts to shape a new product’s profile and condition the expectations of thought leaders in the community are critical steps towards a successful commercial launch.
It’s well-known that biopharma companies may have only one shot at goal in successfully launching a product. Strategic missteps before and during launch could negatively impact the long-term performance of the brand—it will likely be very difficult to recover from these early missteps. In order to ensure a successful launch and maximize the commercial potential of the brand, a structured approach is required around your commercial planning efforts.
Our ProComS Solution will help Maximize the Commercial Potential of Your New Drug
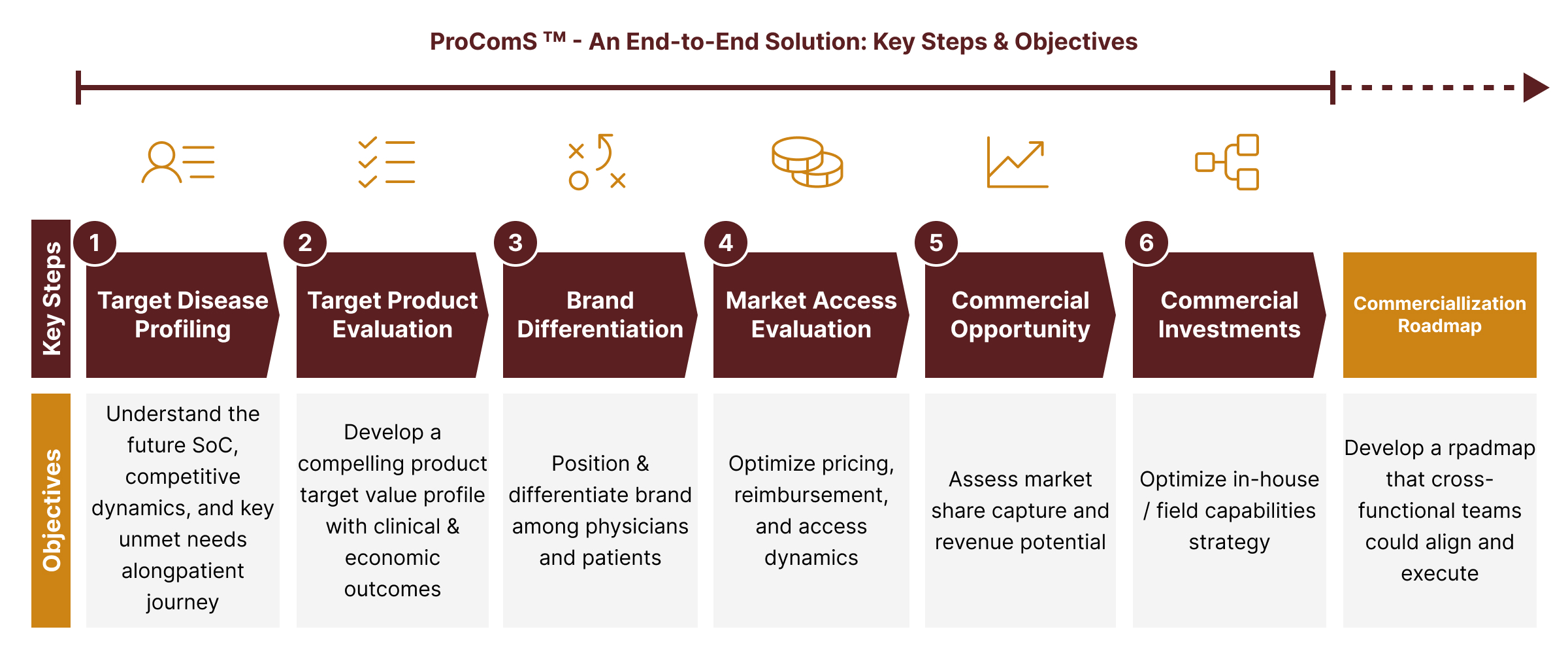
ProComS leverages best practices from previously successful launches to guide biopharma companies on how best to develop impactful commercial strategies, which are then translated into actions that become integral part of the commercial roadmap. Our end-to-end six-step approach outlined below will help uncover and capitalize on new opportunities for your brands in any disease area.
A Proprietary Framework to Drive Global Commercialization through an Integrated Action Plan:
ProComS Focuses on Key Drivers of Commercial Success
Our solution is laser focused on key drivers of product commercialization at the time of launch. It is imperative for biopharma companies to gain a robust understanding of unmet needs in the marketplace – such an effort helps to position and differentiate the brand in the minds of physicians, payers, and patients. We will work closely with your medical and commercial teams to align on where the market is heading and what it will take to succeed in the long run.
Key Areas of Inquiry and Analysis:
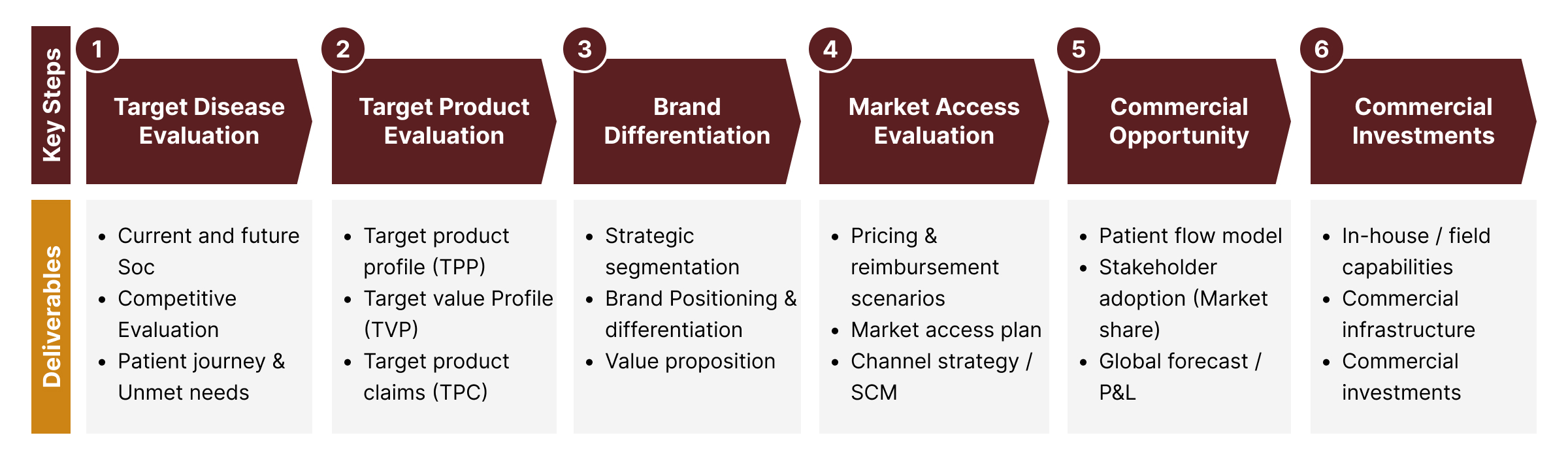
An Insight-driven, Realistic and Actionable Strategic Commercial Plan
ProComS ensures cross-functional alignment around each step (see below) of the commercial road map development process. Executing these steps will create a series of deliverables which then collectively form the basis for mapping out the road to commercial success for your new product(s). The resources required to execute this plan, along with the performance metrics that need to be monitored during the pre-/peri-/post-launch phases, will be identified early for organizational alignment.
Latest Publications on Product Commercialization
Clients and Case Studies
Check out examples of product commercialization work that our team has successfully completed for leading pharma and biotech companies.
RxC International has worked with a number of leading biopharma companies on commercialization initiatives. Our clients range from Fortune 100 companies to small cap companies in the life sciences sector.
RxC has worked with clients from around the world to successfully develop and commercialize dozens of products. Below is a representative list of projects to which we have applied our frameworks.