Monthly Gene Therapy Business Review - November 2017
November saw some important moments in gene therapy. We have summarized the most noteworthy and included the remaining in our news and updates sections.
RxC International reviews gene therapy developments and commercialization efforts on a monthly basis. Sign up here to have our newsletter delivered to your inbox.
1) FDA Accelerates Gene Therapy Process but Warns of Unregulated Risks
Regulators and legislators are balancing speed of approval with warnings about administering unapproved therapies that have not had testing in a regulated environment. This shows an understanding by government entities of the potential benefit from new therapies and the harm from unregulated treatments.
This month, the FDA issued new guidelines to accelerate the approval of gene therapies. The FDA’s announcement came less than a week before Tristan Roberts became the first person to self-inject an untested gene therapy, demonstrating the rise of unregulated gene therapy use among “biohackers” and regenerative medicine clinics. The FDA issued another statement warning against self-administering gene therapies and cautioning against unregulated treatments.
A week prior to Roberts’ action, the Senate Health, Education, Labor and Pensions Committee convened a hearing to learn more about gene therapy and its implications. “While CRISPR and other gene editing technologies could transform human health, it’s not hard to see how we can quickly get into societal and ethical issues,’” said Senator Lamar Alexander. Added Senator Susan Collins, “We live in a global world and it seems like the scientific advancements have outpaced policy in this area. So how do we ensure this exciting breakthrough in gene editing is to be used for good in China, Russia and the U.S.?"
FDA: FDA announces comprehensive regenerative medicine policy framework
S&P Global Market Intelligence: Senators explore gene editing's promise, but raise concerns about misuse
2) Will Gene Therapy Replace the World’s Most Expensive Drug?
Pricing has always been one of the biggest questions in gene therapy’s broad success in the market and will certainly continue to play a significant role in the ability of patients to access treatments. The case of AVXS-101 will test pricing models and the willingness of payers and other entities to pay for combination therapies.
While the gene therapy may carry a hefty price tag, it is a one-time treatment; Spinraza, on the other hand, costs $750,000 for the first year of treatment and hundreds of thousands of dollars for each subsequent year. The biggest question is what an acceptable price?
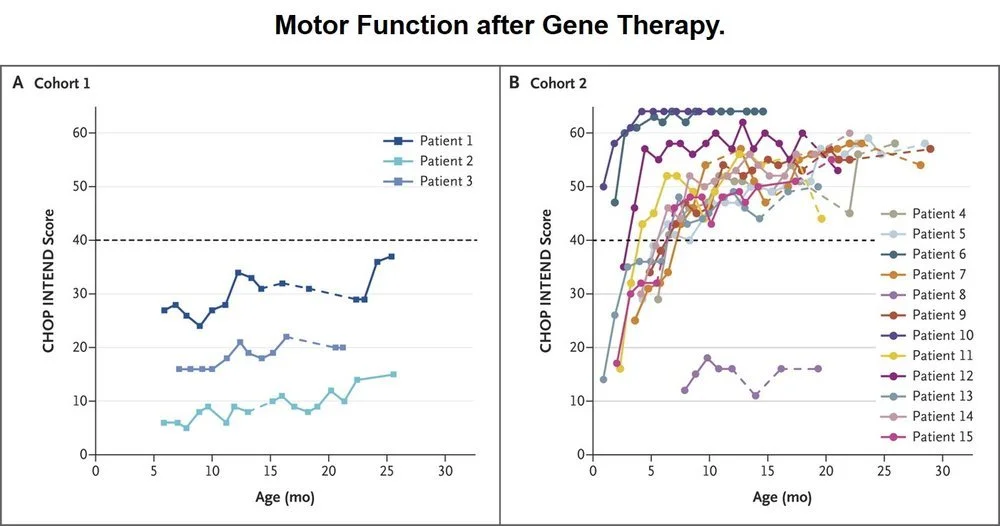
Spinraza, approved last December as the first treatment for spinal muscular atrophy (SMA), may soon have competition from a gene therapy. This month the New England Journal of Medicine published an article detailing success in early clinical trials for AVXS-101, a gene therapy that also treats SMA. Whereas Spinraza forces a gene within the body to produce short motor neurons (SMN), a protein SMA sufferers lack enough of, the new gene therapy replaces the defective gene.
This chart illustrates the Children’s Hospital of Philadelphia (CHOP) Infant Test of Neuromuscular Disorders (INTEND) score for patients in Cohort 1 who did not receive the gene therapy and patients in Cohort 2 who did receive the gene therapy. Source: NEJM
Results from the 15 patients who received the gene therapy were impressive; all were alive at 20 months without needing permanent ventilation, a milestone only 8% of untreated patients reach. Even with these promising results, researchers don’t know whether the positive effects will last and whether a second administration of the gene therapy is even possible.
Newsweek: The Most Expensive Drug in the World is about to be Made Worthless by Gene Therapy
The New England Journal of Medicine: Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy
SMA News Today: Achievements, Hopes and Limits of SMA Treatment: Interview with Spinraza Researcher and Cure SMA
3) Global Treatment Model May Be Necessary for Commercializing Gene Therapies
Rare diseases and specialized skills will require international collaboration for gene therapy success. As this example illustrates, commercializing gene therapies poses challenges that traditional drugs have not faced, including small disease populations, the level of physician expertise required, and special treatment facilities. Questions about where patients receive treatment, who should administer treatment, and, of course, how to pay for these treatments will continue to challenge the development of gene therapies.
Despite uncertainty surrounding internationality and commercialization, this case may be particularly valuable as a blueprint for future gene therapies.
Two years after receiving a life-saving skin grafting procedure, a boy suffering from junctional epidermolysis bullosa (JEB) remains healthy. To treat him, doctors grew nine square feet of new skin, enough to cover over 80% of his body.
Of particular interest is the internationality involved in this case. The child is from Syria but was hospitalized in Germany and treated by Italian doctors granted a compassionate use exception for unapproved treatments. The boy’s new skin was grown in Italy and transported back to Germany where it was grafted onto his body.
Nature International Journal of Science: Regeneration of the entire human epidermis using transgenic stem cells
The Washington Post: Genetically modified skin grown from stem cells saved a 7-year-old boy’s life
The New York Times: Gene Therapy Creates Replacement Skin to Save a Dying Boy
US News: Boy With Rare Disease Gets Brand New Skin With Gene Therapy
4) Gene Editing within the Body: A New Frontier
A breakthrough this month illustrates the potential of non-CRISPR therapies as well as the need for continuing research and development of wide-ranging gene therapy applications and capabilities.
Brian Madeux made history by being the first person to have his genes edited within his body. Madeux, suffering from a genetic disorder called Hunter syndrome, received an injection of zinc-finger nucleases to cut and paste a missing gene into his DNA. If successful, the treatment will enable Madeux’s liver to break down certain molecules and halt the progression of his disease.
While animal testing of this gene therapy were encouraging, the results of Madeux’s treatment will not be known for several months.
Chicago Tribute: U.S. scientists try gene editing inside a person for the first time, aiming to cure a disease
The Atlantic: The First Man to Have His Genes Edited Inside His Body
5) Gene Therapy May Treat Meth Addiction
Unlike cancer gene therapies that turn off defective genes or other therapies that replace faulty genes, the anti-meth therapy introduces a new gene in the body that produces antibodies to assist with warding off chemical addiction. This application of gene therapy technology demonstrates that these therapies are not a uniform group of CRISPR opportunities but are varied and capable of broad application.
A team at the University of Arkansas for Medical Sciences has created a gene that codes an anti-methamphetamine antibody to prevent meth particles from traveling to the brain and creating euphoria. Researchers hope that this breakthrough, combined with behavior therapies, will help meth addicts to overcome the addiction.
MIT Technology Review: Gene Therapy Could Help People Overcome Meth Addiction
Front Line Genomics: Can Gene Therapy End Meth Addiction?
6) Virus Shortage Threatens Gene Therapy Success
A virus shortage highlights the need for companies to have a comprehensive commercial roadmap very early in a new gene therapy’s development or risk being shut out of the industry entirely. With so many companies focused on clinical trials and approval pathways, commercialization issues may fall by the wayside to the detriment of efforts to get much-needed treatments into the hands (or genes) of patients.
The biggest roadblock to successfully developing a gene therapy may not be regulatory or fiscal. The New York Times reports that few companies have the production capability to create viruses necessary for gene therapy delivery and even fewer drug companies have these capabilities in-house. “The result is a logjam. Firms exploring new gene therapies may wait for years in line for bespoke viruses.”
To avoid development delays, forward-thinking gene therapy companies are buying one or more virus production slots years in advance. One biotech company has gone so far as to invest hundreds of millions of dollars to make its own plant for manufacturing viruses. “‘We don’t want to be in a queue, that’s for sure,” said Robert Baffi, head of technical operations at BioMarin.
The New York Times: Gene Therapy Hits a Peculiar Roadblock: A Virus Shortage
Research Updates
1) Encouraging Results for Gene Therapy Treating Heart Failure
A recent study of 13 pigs showed the potential of gene therapy to treat heart failure. Of the 6 pigs who received the gene therapy, heart failure in the left ventricle was reduced by 25% and heart failure in the right ventricle was reduced by 20%; the pigs’ heart sizes were reduced by 10%.
2) Gene Therapy May Treat Rett Syndrome
Researchers have come closer to treating Rett Syndrome, a disease that causes acute impairments affecting speech, mobility, and breathing.
Spectrum News: Scientists move closer to gene therapy for Rett syndrome
3) Novartis partners with Homology Medicines to Evolve Genome Editing Technology
Novartis and Homology Medicines have teamed up to explore the therapeutic potential of adeno-associated viruses (AAVs) as an alternative to CRISPR for some diseases.
4) Canavan Disease Breakthrough Illustrates Complexity of Bringing Gene Therapies to Market
While gene therapy may offer hope for sufferers of Canavan disease, a disorder that damages nerve cells in the brain, the development of a treatment remains a lengthy and complex process.
Springer: Uncoupling N-acetylaspartate from brain pathology: implications for Canavan disease gene therapy
5) Gene Therapy for Young-Onset Parkinson’s in Development
Synpromics and UCL are partnering to develop a gene therapy for young-onset Parkinson’s that focuses on higher-level gene expression using “novel, purpose-designed sequences tailored to different gene expression profiles.”
Fierce Biotech: UCL, Synpromics ally to develop Parkinson’s gene therapy
6) Researchers Discover Key to Regenerating Blood Vessels
Researchers have identified a signal pathway critical to growing new blood vessels from existing ones (angiogenesis).
Science Daily: Key to regenerating blood vessels discovered
7) Phase 1 Parkinson’s Trial Announced
Voyager Therapeutics announced that it is recruiting for Phase 1 trials for VY-AADC01, a gene therapy that codes for the enzyme l-amino acid decarboxylase.
Parkinson’s News Today: Voyager Recruits Patients for Phase 1 Trial Testing VY-AADC01 Gene Therapy Delivery
8) Gene Therapy Shows Promise for Treating Cystic Fibrosis Bacterial Lung Infections
Gene therapy delivery to airways may greatly enhance the treatment success of bacterial lung infections due to cystic fibrosis.
9) Nanoparticles for Death-Induced Gene Therapy to Treat Cancer
A new study indicates tolymeric or inorganic nanoparticles may be the key to gene therapies that kill tumor cells.
10) Study Offers Hope for Relapsed Leukemia Patients
According to trial results published by Stanford, 73% of patients who received a new gene therapy for treatment-resistant B-cell leukemia entered remission.
Other Gene Therapy News
1) Farming Industry Embraces Gene Therapy to Speed Breeding
Gene therapy is extending beyond the pharma industry as a tool used in agriculture to breed for certain traits. Using CRISPR will allow the breeding process to be expedited. “We can do in six months now what used to take us six or seven years,” says James C. Collins Jr., chief operating officer of the agriculture division at DowDuPont.
Wall Street Journal: How Data Science and Gene Editing Will Transform Farming
2) World’s Smallest Tape Recorder
Scientists have modified the bacterium Escherichia coli into a micro tape recorder. “By responding to chemical changes in the surroundings and then 'time-stamping' them in DNA, the technology paves the way for living monitoring devices that could be used in health screens or to analyse pollutants in ecosystems.”
To receive RxC International’s monthly newsletter on gene therapy advancements and commercialization efforts, sign up here.
About RxC International
RxC International is a premier life sciences management consulting firm. RxC collaborates with clients to identify and develop growth opportunities. The firm leverages consulting partners and advisers to combine strategic and operational expertise to bring multiple perspectives to every engagement. The firm has deep expertise in corporate strategy, new product strategy, and commercial excellence.